Zinc Plated Steel
It is valuable to understand the primary component of a galvanized steel coating - zinc. Zinc has been used in construction since 79 AD and its corrosion-protection characteristics for coating steel and iron is well known.
Zinc is essential to all life on Earth; all organisms require it to survive and complete basic biological functions. As the 27th most abundant element on Earth, zinc is naturally found in rocks, soil, air, water, plants, and animals. Read more about the function of zinc in the body.
As with all metals, zinc corrodes when exposed to the atmosphere. Zinc has the ability to form dense, adherent corrosion by products, thus the rate of corrosion is dramatically slower than other ferrous metals (10 to 100 times slower depending on the environment). The byproducts of zinc corrosion naturally form on the surface of zinc plated metals as the coating is exposed to water and air. These by-products are known as the "zinc patina" or white rust. The zinc patina is an additional barrier between the steel and corrosive environment. Read more about zinc corrosion.
Although there are many zinc coatings broadly termed as "galvanizing," each has distinct properties. These various properties influence galvanized steel suitability, as well as the economics and performance in the environment over time. The way in which each zinc coating is applied, how it bonds to the base metal, its hardness, corrosion resistance, and thickness (figure 1) varies. (6)
Learn about hot-dip galvanizing here & electrogalvanizing here - the zinc coatings used to galvanize our steel buckets.
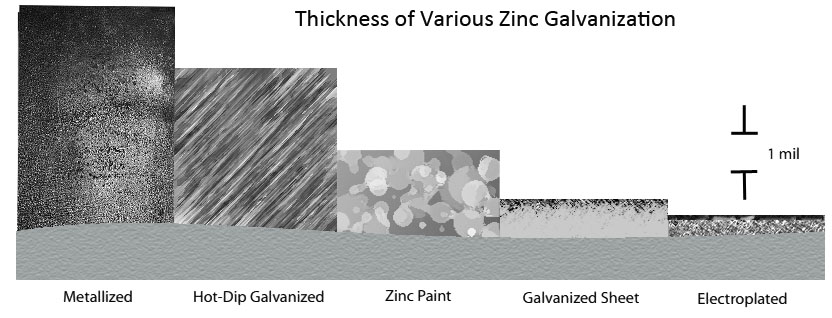
Figure 1: Thickness and microstructure of various zinc coatings (6 p.1)



